Approved US patent for MedVasc’s instrument for varicose vein surgery
 Michael Åkesson, CEO of MedVasc
Michael Åkesson, CEO of MedVascMedtech company MedVasc’s innovative instrument for varicose vein surgery has received patent protection in several countries – most recently from the US. This is a highly significant milestone for MedVasc and for the company’s strategy to be active in key markets, according to the company’s founder and CEO, Dr. Michael Åkesson.
About 30 percent of the population worldwide suffers from varicose veins. Laser treatment, which is currently the most commonly used treatment for varicose veins, entails pain and discomfort for patients. The pain arises when local anesthesia is administered prior to the procedure, with up to 30 injections through the skin along the vein of the leg to be treated.
MedVasc’s founder Dr. Michael Åkesson is a practicing surgeon who has developed a technique in which laser treatment can be carried out with minimal pain. Using the technique, which is called Solutio™, the laser fiber and the Solutio™ catheter are connected and inserted into the varicose vein to be treated.
Once in place in the varicose vein, local anesthesia is administered from inside the vein through the Solutio™ catheter, which contains a bent needle that penetrates the vessel wall from inside the vessel where it can then deliver local anesthetic out into the surrounding tissue. The patient does not feel the needle puncture because there are no pain receptors on the inside of the vessel wall.
“The US market will be highly significant for us, so we are extremely pleased that the patent was now approved in the US. The US patent authority, which is known for its stringency, has now given us approval to use our medical device for treatment of ‘defective blood vessels, body cavities and canals’,” says Dr, Michael Åkesson, CEO and founder of the company.
The instrument has already been approved in Sweden, Australia, Russia and South Africa, and the company expects to receive approval for the entire EU shortly.
MedVasc is a member of SmiLe Incubator and a portfolio company under LU Holding, Lund University’s holding company.
For more information, please contact:
Michael Åkesson, CEO MedVasc AB, michael.akesson@medvasc.se; mobile +46705578131, www.medvasc.se
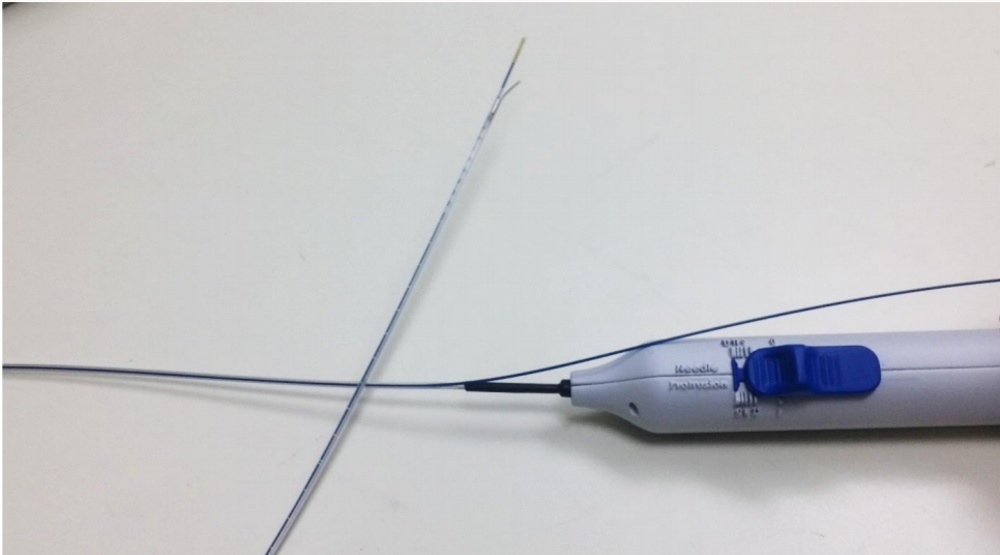
Image of the Solutio catheter showing the control handle with the blue slider that controls the needle at the other end of the catheter. Here the needle is in the extended position whereupon it deviates to the side, away from the blue laser fiber that is connected to the Solutio catheter. The bent needle deviates and penetrates the vessel wall so that the anesthetic can be applied outside the vessel through the needle.
About SmiLe Venture Hub – Pioneering Life Science and Foodtech Innovations
SmiLe Incubator is a life science business incubator based in Medicon Village in Lund. SmiLe helps entrepreneurs to commercialize their ideas. There are currently 25 companies in SmiLe, which together with alumni companies, have attracted more than EUR 380 million in venture capital to date since 2014. SmiLe offers business coaching, a large network of contacts and a dynamic community, as well as well-equipped laboratories which is unique of its kind in Sweden. SmiLe is a non-profit organization and receives basic funding from Region Skåne, Lund Municipality, Lund University and Medicon Village. SmiLe´s sponsors are Agilent, Sparbanken Skåne, Awa, Høiberg, Prevas, Setterwalls, Zacco, Aqilion. SmiLe’s listed alumni companies have a market capitalization of EUR 1,28 billion (Q3 2020). www.smileincubator.life