New instrument for varicose vein surgery produces promising results in clinical study
The medical device company Medvasc reports promising results from an initial clinical study in which patients with varicose veins were treated with a new, pain-free and effective instrument, the Solutio™ catheter, which was developed by the company. The company is a member of SmiLe Incubator in Medicon Village and a portfolio company under LU Holding, Lund University’s holding company.
All patients in the study reported that pain was minimal, and the Solutio™ catheter was shown to be safe and effective without complications. Additional studies are planned during the year to verify the promising results, to be followed by preparations for a product launch.
--------
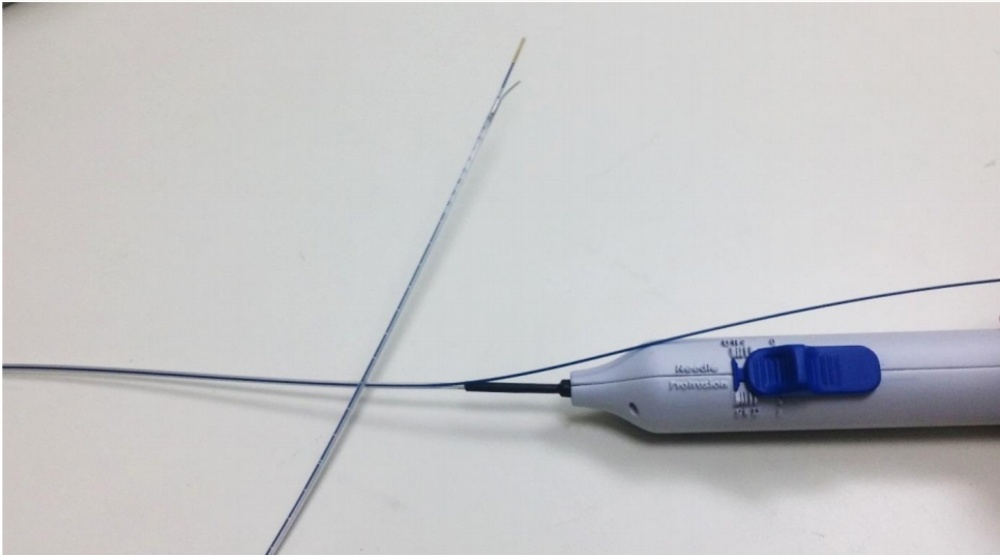
Image of the Solutio catheter showing the control handle with the blue slider that controls the needle at the other end of the catheter. Here the needle is in the extended position and then deviates to the side away from the blue laser fiber that is connected to the Solutio catheter. Because the needle is bent, it deviates through the vessel wall so that anesthetic can be applied outside the vessel through the needle.
Laser treatment is currently the leading treatment technique for varicose veins. The downside of this technique is that it is painful. Extensive local anesthesia is often required, with up to 30 injections through the skin along the vein of the leg to be treated, as well as both sedatives and analgesics during the procedure. It is painful for the patient and resource-intensive for the healthcare organization.
About 30% of the population suffer from varicose veins, but only a small number of patients are treated. Untreated, varicose veins may cause chronic leg ulcers, which are extremely expensive for the healthcare system. Medvasc has high hopes that the promising clinical results will result in treatment of more patients for varicose veins.
Medvasc has developed a technique in which laser treatment can be carried out with minimal pain: Using the Solutio™technique, the laser fiber and the Solutio™catheter are connected and inserted into the varicose vein to be treated. Once in place in the varicose vein, local anesthesia is administered from inside the vein through the Solutio™catheter, which contains a bent needle that can penetrate the vessel wall from inside the vessel out into the surrounding tissue, where the local anesthetic is administered.
The patient does not feel the needle puncture because there are no pain receptors on the inside of the vessel wall. Using Solutio™ reduces the painful insertion of the needle through the skin to only one, which minimizes the patient pain associated with laser treatment.
“Many people suffer from varicose veins and since only a few patients are treated, many others suffer from complications such as pain and leg ulcers that are difficult for the patient and have a negative impact on health services. Solving the problem of pain during treatment will be an important asset in the treatment of varicose veins. The promising results from the study indicate that a solution is close at hand,” says Assistant Professor Anders Lundell, from the Scandinavian Venous Center in Malmö. Dr Anders Lundell was the principal investigator in the study.
Michael Åkesson, a physician as well as the CEO and founder of the company says:
“The results from this study are extremely promising and, in the future, the Solutio™ technique will be able to solve the problem of pain during laser treatment, and the healthcare system will be able to offer patients the best scientifically proven and most effective technique for treatment of varicose veins.”
The goal of the present study was to evaluate pain and safety associated with the Solutio™technique. Nine patients who met defined inclusion criteria participated in the study and had the procedure with the combined laser Solutio™technique. Pain was assessed using the Visual Analog Scale (VAS), in which maximum pain is assigned a value of 100. All patients in the study reported minimal pain ranging between 5 and 24 according to the VAS. Treating physicians also considered the Solutio catheter to be safe and easy to use.
MEDVasc’s product launch plans include conducting another clinical study during the year to verify the earlier study using an updated Solutio™ prototype. The application process for CE marking in Europe and the US has been initiated and the company entered into discussions with partners regarding manufacturing and production of the Solutio™ catheter. Sales will involve disposable kits delivered directly to treating clinics and doctors. The product launch is planned for 2022, beginning with the Nordic market and followed by the EU and the US.
For more information, please contact:
Ingela Hallberg, Project manager, clinical research and development MedVasc AB, ingela.hallberg@medvasc.se; mobile +46704204500
Michael Åkesson, CEO MedVasc AB, michael.akesson@medvasc.se; mobile +46705578131
About SmiLe Venture Hub – Pioneering Life Science and Foodtech Innovations
SmiLe enables a future of better healthcare by building a community of world-class life science innovators. Located in Medicon Village, a life science cluster in Lund, Sweden, SmiLe facilitates startups looking to develop next-generation healthcare and life sciences technologies. By bringing together life-science entrepreneurs, investors, scientists, the university and the municipality, we create an attractive, forward-thinking atmosphere that enables creativity and smart science to thrive. Being a not-for-profit organization gives companies the security of knowing that we work only in the interests of the entrepreneurs we sponsor.